Posted May 31, 2021: by Bill Sardi
Prior Negative COVID-19 Vitamin C Study, Halted Prematurely Because of Alleged Ineffectiveness, Found to Increase Rate of Recovery by 70% Upon Reanalysis
Where was the peer review? Where were the trained medical journalists to take researchers to task over prematurely halting a vitamin C/COVID-19 symptom study when both statistical and meaningfully clinical benefits were readily apparent, when so many American lives were reported to be at stake?
On February 12, 2021 MedPageToday.com slammed vitamin C for “not living up to its hype,” resulting in researchers halting the study prematurely after only 214 COVID-19 diagnosed patients were enrolled in a 520-patient study (JAMA Open Network 2021). Investigators inexplicably discontinued enrollment errantly believing it would have been a futile effort to continue. News media carried this as a headline story throughout the world – vitamin C had failed once again!
But three months later scientific reviewers report in the journal Frontiers in Immunology what that vitamin C study actually reported before its censors and interpreters maligned it :
The reviewers write: “It was illogical to have stopped the trial early because of futility.” The study investigators were taken to task for considering a 1.0-day reduction in symptoms duration is clinically important, “yet on the other hand deemed a 1.2-day reduction was futile and justified early termination of the study.”
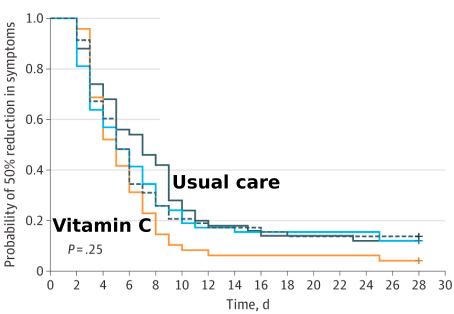
The team of reviewers also found:
Vitamin C researchers Steve Hickey and Hilary Roberts of Manchester, England, claim there is a huge difference between the use of vitamin C to prevent a common cold (~10,000 milligrams/day in divided doses) vs. treating a cold (could require 50-100 grams (50,000 to 100,000 milligrams). Other researchers have found that white blood cells have blood concentrations that are at least 14-fold higher than blood plasma, which is the standard way to measure blood vitamin C levels.
Historically, an early error was that researchers mistakenly believed no vitamin C is absorbed beyond 200 milligrams/dose. During illness vitamin C is rapidly depleted and doses up to 200 grams (200,000 milligrams) may be needed. Hickey and Roberts write that the dose of vitamin C needed to treat a cold is “perhaps a minimum of 10 grams (10,000 milligrams) of oral vitamin, followed by a least 2 grams each hour.”
The preemptive cancellation of the vitamin C/COVID-19 symptom study was not just an error, it was an example of blatant scientific bias with complicity by peer reviewers and failure to critique obvious research errors by the news media. The research report published in JAMA Network Open should be retracted
The truth will eventually prevail, but unfortunately, not enough people are likely to hear about it.
Posted in Vitamins ; No Comments »
11
17
52
95
14
24
237
6
56
43
10
116
15
66
105